4.1 Functional Groups of the Carboxylic Acids and Their Derivatives
We introduced the carbonyl group (C=O)—the functional group of aldehydes and ketones—in Chapter 3 "Aldehydes, Ketones". The carbonyl group is also found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group.
A carboxylic acid is an organic compound that has a carboxyl group. The carboxyl group is a functional group that contains a carbon-oxygen double bond and an OH group also attached to the same carbon atom, but it has characteristic properties of its own. As with aldehydes and ketones, carboxylic acid formulas can be written to show the carbon-to-oxygen double bond explicitly, or the carboxyl group can be written in condensed form on one line. In general, carboxylic acids are represented by the formula RCOOH, where R is a hydrocarbon group.
Esters are represented by the formula RCOOR’, where R and R’ are hydrocarbon groups. The ester, which is organic compound derived from a carboxylic acid and an alcohol in which the OH of the acid is replaced by an OR group, looks somewhat like an ether and also somewhat like a carboxylic acid. Even so, compounds in this group react neither like carboxylic acids nor like ethers; they make up a distinctive family. Unlike ethers, esters have a carbonyl group. Unlike carboxylic acids, esters have no acidic hydrogen atom; they have a hydrocarbon group in its place.
An amine is a compound derived from ammonia (NH3); it has one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. Like NH3, amines are weak bases. The functional group of an amine is a nitrogen atom with a lone pair of electrons and with one, two, or three alkyl or aryl groups attached.
The amide functional group has a carbonyl group joined to a nitrogen atom from ammonia or an amine. The properties of the amide functional group differ from those of the simple carbonyl group, NH3, and amines.
Esters and amides are considered to be derivatives of carboxylic acids because the OH in the carboxyl group is replaced with another group. These functional groups are listed in Table 4.1 "Organic Acids, Bases, and Acid Derivatives", along with an example (identified by common and International Union of Pure and Applied Chemistry [IUPAC] names) for each type of compound.
Answers
1.
a.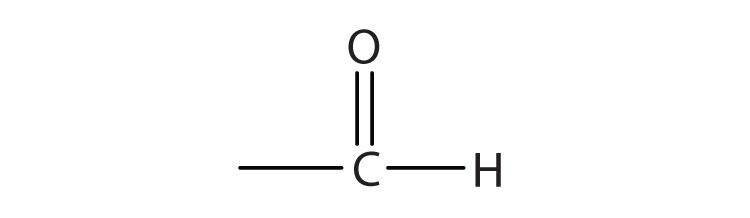
b.
c.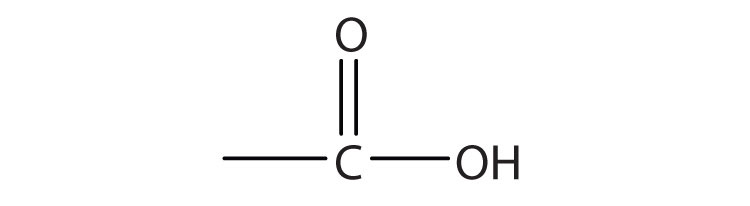
3.
a.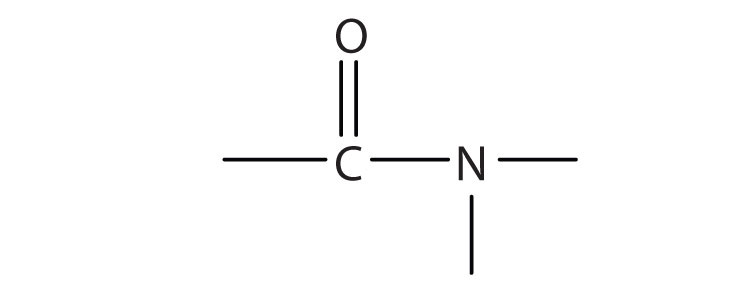
b.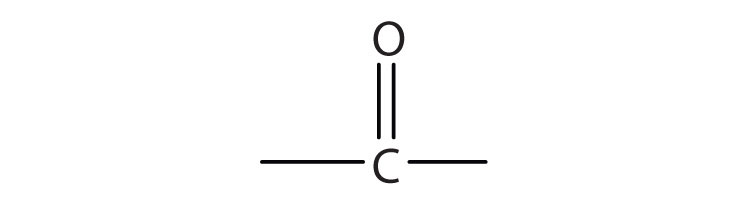
c.


